
/sublimation-of-iodine-solid-iodine-changes-directly-from-solid-to-gas-and-recrystallizes-on-glass-h-139822531-574f55335f9b5892e867db16.jpg)
For example, the stainless steel reactor vessel, soil in the sub-surface and particulates in the atmosphere. In the event of a nuclear incident and subsequent release of radioactive iodine, such as the Fukushima accident, iodine-containing species could potentially interact with a number of surfaces causing further molecular interconversions. In the atmosphere, a wide variety of chemical forms are observed due to radical pathways that can be initiated by interaction with light, whereas in the solution phase, iodide (I −), iodate ( I O 3 -) and molecular iodine (I 2) are the most common species ( Pourbaix, 1974). Iodine can change between oxidation states either by chemical reaction or disproportionation in both the gas and solution phases ( Wren et al., 1986 Saiz-Lopez et al., 2012 Gómez Martín et al., 2022). Iodine, unlike other common fission products such as noble gases, is challenging because of the wide range of molecular forms and oxidation states (from +7 in IF 7 to −1 in hydroiodic acid) that can be adopted. Capture materials, such as activated carbon, are used routinely in nuclear power plants ( Riley et al., 2016 Vellingiri et al., 2018) and medical isotope production facilities ( Doll et al., 2014) to remove this hazard during normal operations to reduce emissions to acceptable levels.
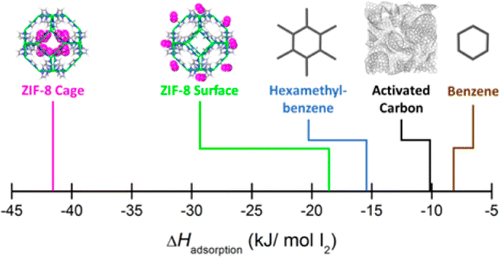
The short-lived radioactive isotope, 131I, with a half-life of around 8 days, would be a particular health concern if released into the environment. Iodine is a common fission product resulting from the transmutation of uranium fuel in nuclear power reactors. In all cases we found no significant charge transfer between the iodine species and the graphite, thus we conclude that all the iodine species studied undergo strong physisorption to the graphite. For each species the relative binding energies spanned the range of 21–33 kJ mol -1 and graphite-iodine distance was in the range of 3.52–3.93 Å. It was found that for molecular iodine, the iodine atoms tended to either situate above the center of a hexagonal site on the graphite or directly atop a carbon atom with the lighter components resting closer to the graphite. The PBE exchange-correlation functional with D3 dispersion was employed. In this work we perform an ab initio study of the adsorption onto the surface of a graphite sheet of I 2, CH 3I, and inorganic acidic iodine species (HI, HOI, HIO 2, and HIO 3), which were selected to examine the possible effect of oxidation state on adsorption. Previous studies have used DFT and the graphite (0001) surface as a surrogate for adsorption, those studies focus on the species I Iodine can be captured using a number of materials and frequently, this is accomplished with activated carbon impregnated with organic bases. The release of 131I is of particular concern to human health. In the event of a nuclear accident, fission products may be released into the environment. 2Physical and Computational Sciences Directorate, Pacific Northwest National Laboratory, Richland, WA, United States.1National Security Directorate, Pacific Northwest National Laboratory, Richland, WA, United States.


 0 kommentar(er)
0 kommentar(er)
